Temperature Controlled Logistics for Cold Chain Management

By T.C. Baker
Companies shipping medicines and other patient-critical products are doubling down on temperature-controlled logistics (TCL) for their cold chain management, we found in a recent survey. That’s probably because the environment in which life science companies operate is increasingly complex, being driven by a more and more demanding healthcare agenda. The global need for innovative, cost-effective medicines continues to rise. At the same time, patients are demanding greater value for money, proven effectiveness of products, and access to information.
Temperature Controlled Logistics Survey Results
To meet these demands, healthcare companies are seeking ways to improve supply chain visibility for better TCL management. Nearly 60% of respondents in the Pharma Logistics IQ-TransVoyant survey invest in end-to-end visibility and almost 40% have fully operational solutions.
However, this contrasts with other survey responses, where respondents cited challenges such as a lack of willingness to spend money:
- 39% said budget constraints were the main challenge in implementing a digital solution
- 25% faced obstacles in building a business case for digital cold chain solution
The Rise of Digital Temperature Controlled Logistics
Investment in end-to-end visibility has historically tended to be low, making achieving true supply chain transparency challenging. But with new digital supply chain management solutions, that lack of investment is slowly changing.
The charge is being led by pharmaceutical companies. The risks inherent with pharma cold chain shipping and the security of medicines while in transit create supply chain management implementation complexity. Critical parcels like medicines require 24/7 monitoring to ensure patients’ safety and product quality.
The solution? To secure the uninterrupted supply of products around the world, global supply chain leaders in the pharma industry are increasingly utilizing digitization and investing in key logistics technologies (i.e., control towers, Internet of Things, and route-to-market or RTM).
How TCL Digitization Works
Digitizing temperature-controlled logistics can provide supply chain leaders with a common operating picture of the life and condition of all customer orders. This gives internal teams visibility to time delays, product condition, and external events. The result is continuous improvement of patient safety, logistics planning and execution, and eliminating discards.
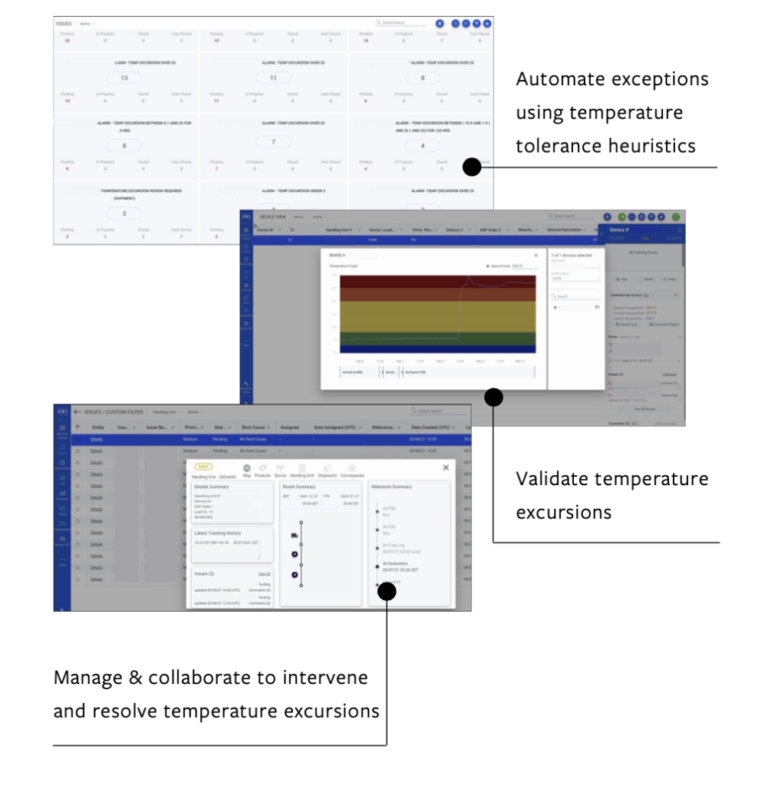
Figure 1: Digital processes for temperature-controlled logistics can identify, validate, and even intervene to prevent or mitigate excursions for sensitive products.
Leveraging TCL capabilities, one global pharmaceutical company that TransVoyant worked with conducted enough temperature excursion interventions to prevent over one million discards of medicine doses. Their team was able to develop a TCL solution for global delivery and quality business divisions via automation, exception management, analytics, and reporting. Then the team could respond in real time to mitigate issues and save product.
Key program features included:
- automated reporting for SOP compliance and adherence to carrier, lane, route, and thermo-packaging units
- seamless end-to-end connection of order, shipment, IoT, carrier, mode, and external event information
- machine learning (ML) predictive insight and automation providing daily operational intelligence
By leveraging these tools, pharmaceutical companies can transform and automate their entire shipment intervention process, benefiting quality and audit team workloads, reducing manual reporting claims, and lowering insurance premiums.
T.C. Baker is Vice President, Solution Consulting at TransVoyant.



